Which phrase identifies and describes this reaction. Cs 02g C02g B.

This Self Made Cartoon Describes That The Duty Of A Chemist To Research How Water Impacts Ecosystems A Water Pollution Solutions Water Pollution Precipitation
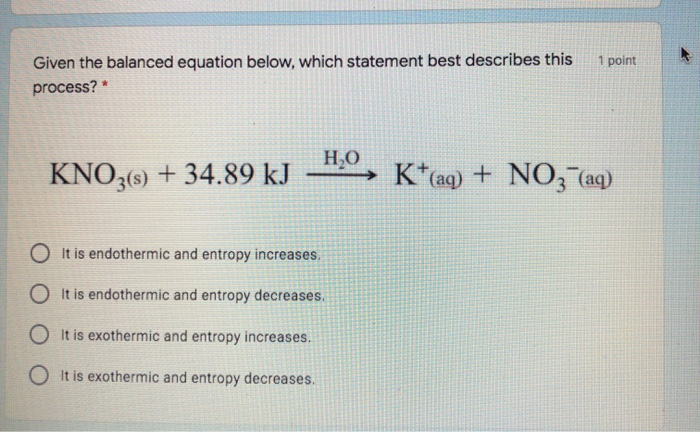
Given the balanced equation.

. 10Given the balanced equation. 1 point Given the balanced equation below representing a reaction which statement explains why the energy term is written to the right of the arrow. 25Which balanced equation represents nuclear fusion.
It is exothermic and entropy increases. B It is endothermic and entropy decreases. Cu S Cus energy O The compound Cus is composed of two metals.
It is endotherm increase 2 It is endothermic and entropy decreases. The balanced equation of the chemical reaction is given below. Which statement best describes this process.
Up to 24 cash back 21 Given the balanced equation representing a reaction. It is exothermic and entropy increases. 1fission mass converted to energy 2fission energy converted to mass 3fusion mass converted to energy 4fusion energy converted to mass 26Given the balanced equation representing a nuclear reaction.
The dissolving of the LiBr s in water is an endothermic process. 21H 31H 42He 10n Which phrase identifies and describes this reaction. H2O heat H2Og In the box below using the key draw a particle diagram to represent at least five molecules of the product of this physical change at 120C.
2 Energy is absorbed and a bond is formed. 2 A bond is formed and energy is released. 4 Energy is absorbed as bonds are formed.
It is endothermic and etffipy increases. 22 A molecule of butane and a molecule of. KN03s 3489k J Kaq NC3 aq Which statement best describes this process.
1 A bond is formed as energy is absorbed. Br 2 energy Br Br Which statement describes the energy change and bonds in this reaction. Another possible reaction of U-235 is represented by the incomplete balanced equation below.
CH4g 202g cozg 2H20O C. 4 Energy is released and a bond is formed. The activation energy is.
A It is endothermic and entropy increases. The activation energy is 50kJ and the reaction is exothermic. Cl 2g Clg Clg What occurs during this change.
It is exothermic and AH equals 918 kJ. It is exothermic and entropy decreases. Given the balanced equation representing a reaction at 1013 kPa and 298 K.
This process occurring at standard pressure is represented by the balanced equation below. A The entropy of the LiBraq is greater than the entropy of the water. It is endothermic and entropy decreases.
CoI2 aq Pb NO32 aq PbI2 s Co NO32 aq Give the balanced ionic equation for the reaction. Which balanced equation represents an endothermic reaction. B It is endothermic and entropy decreases.
CH4g 2O2g -- 2H2Og CO2g heat Which statement is true about energy in this reaction. Kinetics Equilibrium Practice Test Date. 2 Energy is released as bonds are formed.
Which factors must be equal in a reversible chemical reaction at equilibrium. 1 Energy is released as bonds are broken. Fusion of hydrogen energy comes from mass.
Given the balanced equation. Which statement correctly describes the energy changes that occur in the forward reaction. Cl2g 2KBraq 2KClaq Br2l According to the above equation it can be said that chlorine in its gaseous form Cl2 reacts with pottasium bromide reactants to form pottasium chloride KCl and bromine which is a liquid at room temperature.
The dissolving of the LiBr s in water is an exothermic process. KNO3 s 3489 kJ H20 K aq NO3 - aq Which statement best describes this process. CH4g 202g 3H2g 2NH3g D.
3 Energy is released and a bond is broken. Which statement best describes this process. It is endothermic and entropy increases.
D It is exothermic and entropy decreases. Given the balanced equation. 3 A bond is broken as energy is absorbed.
It is endothermic and entropy decreases. 1 fission mass converted to energy 2 fission energy converted to mass 3 fusion mass converted to energy 4 fusion energy converted to mass. Given the balanced equation representing a reaction.
Hao KNOBs 3489 N03-aq Which statement best describes this process. C It is exothermic and entropy increases. Up to 24 cash back 22Given the balanced equation.
1 Energy is absorbed and a bond is broken. Given the balanced equation representing a reaction. D The dissolving of the LiBrs in water is an exothermic process.
The activation energy is 50kJ and the reaction is endothermic. 3H2g Given the balanced equation. C It is exothermic and entropy increases.
I I I2 Which statement describes the process represented by this equation. The reaction of aqueous cobalt II iodide and aqueous lead II nitrate is represented by the balanced formula equation. D It is exothermic and entropy decreases.
A It is endothermic and entropy increases. A It is endothermic and entropy increases. It is endothermic and AH equals 918 kJ.
B The entropy of the LiBraq is less than the entropy of the water. Given the balanced equation. 3 Energy is absorbed as bonds are broken.
CH4 g 2O2 g 2H2O g CO2 g heat. O Energy is absorbed as the bonds in Cus form. 12 Given the balanced equation representing a reaction.
C The dissolving of the LiBrs in water is an endothermic process. Form many hydronium ions. HO KN03s 3489 N03-aq Which statement best describes this process.
Graph 2 shows the relationship between pH value and hydronium ion concentration for common aqueous solutions and mixtures. The entropy of the LiBr aq is greater than the entropy of the water. It is exothermic and entropy decreases 5.
11 0 KN03s 3489 NOa-aq Which statement best describes this process. It is exothermic and AH equals 918 kJ. It is exothermic and entropy increases.
It is endothermic and AH equals 918 kJ. Which balanced equation represents an endothermic reaction. Given the balanced equation.
AgNO3 aq NaCl aq - NaNO3 aq AgCl s This reaction is classified as. Which of the following is a correctly balanced equation for a reaction between hydrogen gas. Base your answers to questions 14 through 16 on the information below.
23592U 10n 9236Kr 14256Ba 210n energy For this reaction the sum of the masses of the products is slightly less than the sum of the masses of the reactants. 2NOg Given the balanced equation. A thermometer is in a beaker of water.
N2g 3H2g 2NH3g 918kJ ch statement is true about this reaction. O The compound Cus is composed of two nonmetals. The reaction is exothermic because it releases heat.
49 Given the balanced equation representing a nuclear reaction. It is endothermic and entropy decreases. The entropy of the LiBr aq is less than the entropy of the water.
Solved 1 Point Given The Balanced Equation Below Which Chegg Com

Factor That Influence The Entropy Of A System Teaching Chemistry Entropy Molar Mass

0 Comments